Methodology
LYSE-IT METHODOLOGY
Lysis, the process by which a bacteria or virus is “opened-up” to release its genetic material is a common biochemical technique practiced by a great many researchers daily and is crucial as the first step for the detection & identification of many bacteria and viruses. Lysing is typically undertaken by adding a chemical cocktail to the sample (lysis buffer) accompanied by sample heating. This process traditionally takes several hours to complete and often involves expensive lysis buffers, where different buffers are often needed for different sample / media types. In addition, further sample processing steps are then often needed to fragment the Lysate DNA, RNA or denature the released cellular proteins, using further kits and ultimately increasing the cost and time needed for sample preparation.
To address this bottleneck of speed, cost, complexity and the fact that many laboratories employ multiple kits for routine testing, Lyse-It Llc. has recently launched Lyse-It™, a means to lyse virtually any cell, spore or virus rapidly (typically < 30 seconds) in a single-step on a single platform, thereby enabling the genetic material (e.g. DNA) to be collected for downstream analysis on any platform. A significant advantage of the Lyse-It technology is that in addition to Cell lysing, DNA / RNA fragmentation can occur simultaneously (at later lysing times), readying your lysate for immediate analysis and removing the need for a second DNA / RNA fragmentation step.
At early lysing times (typically less than 15 seconds), cells are simply lysed, releasing all the intact biomolecules into solution, ideal for proteomics and large molecular weight DNA sequencing. Thus, the Lyse-It platform gives the customer the tunablility of simply lysing cells, or at later times (30 seconds), both lysing cells and simultaneously fragmenting the DNA/RNA.
Cellular Membrane Disruption
Lyse-It is ideal for disrupting many different cell and bacterial types. Figure 1 shows Bacillus Anthraces (Anthrax) spores both before and after lysing. Under conditions of 30% power for nearly 60 seconds, > 90 % of the spores were lysed with only a few bacteria remaining, noting the disruption of the exosporium. The lysate was subsequently used for the low copy detection of Bacillus Anthraces [1].
Figure 1 – TEM images of the lysing of Bacillus Anthraces spores (Anthrax) using Lyse-It. Left - Before lysing; Right - Post lysing.
Similarly, Figure 2 shows TEM images of the lysing of Chlamydia Trachomatis using 30% power for 30 seconds, in our 1 ml sample chambers, volume = 500 µL. As we can see from the Right hand image, all of the Chlamydia have been lysed, with the DNA < 500 bp in size (confirmed by gel electrophoresis), which was ideal for the molecular detection of the Chlamydia using both PCR and MAMEF [2].
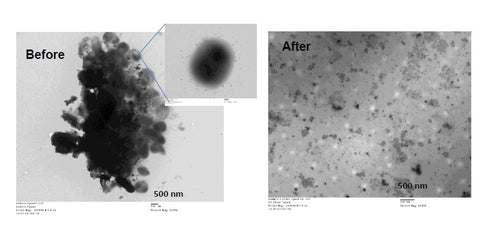
Figure 2 – TEM images of Chlamydia both before (Left) and after lysing (Right) using Lyse-It.
DNA Fragmentation
In addition to rapidly lysing “opening” cells, Lyse-It has the significant advantage of simultaneously fragmenting DNA and RNA, where both the microwave power and length of time lysed, both progressively continue to cut DNA and RNA to smaller and smaller fragment sizes. This significant advantage allows users to “tune” base pair sizes to their desired lengths (distribution of sizes).
Figure 3 shows the DNA fragmentation of Neisseria gonorrhoeae using Lyse-It for different microwave lysing powers, as compared to using simple microwave heating, i.e. without Lyse-It. In this figure we additionally compare our 1 and 5 ml lysing chambers for different heating times, namely, 30, 60 and 90 seconds. U – corresponds to Unlysed sample, M – DNA Ladder. As we can see from the figure, Lyse-It significantly fragments the Neisseria gonorrhoeae DNA, with the DNA becoming progressively fragmented at longer times and higher powers, see ref [3].
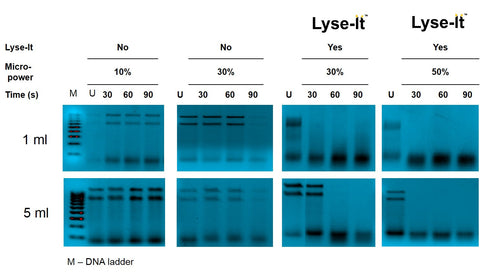
Figure 3 – DNA fragmentation patterns of N gonorrhoeae both with and without Lyse-It, for different sample volumes, microwave powers and lysing times.
Dynamic Lysing Range
Another significant feature of the Lyse-It technology is that it can lyse cells over a very large CFU range (concentration / number) of the cells or bacteria. Table 1 shows CFUs ranging from 0.01 to 1 million colony forming units. When lysed at 30% power for 30 seconds (500µL in a 1 ml sample chamber), no Chlamydia grew, the TEM images additionally confirming 100 % lysing efficiency, Figure 2. Moreover, in a recent paper, Lyse-It has been shown to efficiently lyse a solution of 10^9 algae to release the lipids, for bio-diesel applications [4]. Please see our Facebook and Twitter pages for more examples and applications.
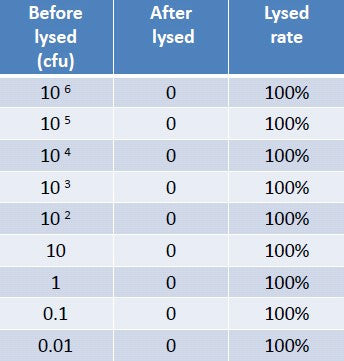
Table 1 – Lysing efficiency of a large concentration range (CFU’s) of Chlamydia Trachomatis.
Lysing of Food Substances – Intact Protein Collection
Lyse-It is also ideal for extracting larger molecular weight DNA and intact proteins from food substances, as well as bacteria, viruses, spores and plant/human cells. Figure 4 shows the SDS PAGE of Spinach lysate, comparing Lyse-It to conventional heating for lysis. As we can see, Lyse-It is able to release a significant amount of protein at early lysing times (ideal for proteomics), and even degrade the protein as needed at longer heating times and higher powers, offering customer flexibility in the extent of lysing. Interestingly, proteases and nucleases are also degraded at longer lysing times. Please see both our Facebook and Twitter pages for more examples and applications.

Figure 4 - SDS PAGE of Spinach lysed by conventional heating and with Lyse-It for different lysing times and microwave powers. At early times, much more intact protein is observed in the lysates Vs standard lysing approaches, which is ideal for proteomics and ELISA-type applications. At longer lysing times and higher lysing powers, proteins, DNA/RNA, proteases and nucleases are fragmented.
Standard Lysing Conditions
30 % Microwave cavity power and 30 seconds lysing time is a standard setting for Lyse-It. During this 30 second lysing procedure, a 1 ml Deionized Water sample is heated to between 50 – 60 °C, which is generally adequate to lyse most gram negative bacteria, as well as fragment a significant amount of genomic DNA < 1000 bp sizes. Please note: this temperature is a simply a calibration temperature for the microwave device and slides. Lysis on the Lyse-It platform actually works by the non-thermal effects of water expansion and the generation of reactive oxygen species. We recommend you undertake both lysing for longer and at higher powers for both gram positive bacteria and also for shorter base-pair size distributions. Please see the next section, “Lysing Temperature”, for further details.
Lysing Temperature
Both the Lysing of cells and the simultaneous DNA fragmentation patterns (distribution of base-pair sizes) are simply a function of both lysing time and microwave power. Table 2 shows the typical mean temperatures achieved and the relevant settings you may want to consider for your lysing / DNA bp-size needs.
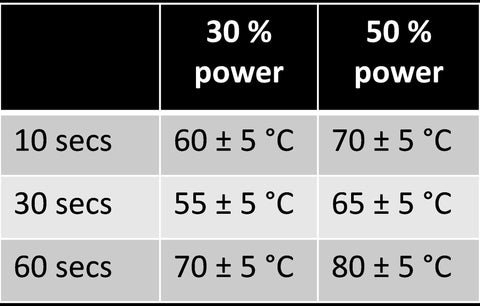
Table 2 – Approximate temperatures for different lysing times and microwave powers. Data recorded for 1 ml DI water in a 1ml sample chamber. These values can be used as a means to check your monthly calibration of the microwave and lyse-It slides.
Lyse-It Slides
The Lyse-It slides are used to focus the microwave energy generated by the microwave oven into your sample chamber. It is the focusing of the microwave energy that rapidly lyses cells and fragments DNA / RNA, by both the rapid expansion of water and the generation of reactive oxygen species, respectively. The Lyse-It slides must only be used with the triangles on the top of the slide, which is depicted by either a sticker or lettering on the slide “UP” or “Lyse-It”. These slides are single use and are destroyed after a single use. Lyse-It slides are sold in 10, 25, 50 and 100 packs.
Black Slide Mount
The Lyse-It slides are mounted into the slide mount by simply sliding them into the groove. The slide mount fits into the center of the microwave oven. The slide mount is reusable over a great many runs, but may eventually degrade over time depending on the extent and rate of heating that you apply.
Sample Chambers
We currently offer sample chambers for 1, 3 and 5 ml maximum volumes. When the paper is peeled from the back of the sample chambers an adhesive layer is exposed. This allows the sample chambers to be firmly pressed onto the Lyse-It slides to make a sample chamber. Care must be taken to ensure that no gaps between the sample chamber and glass are evident, else sample will leak onto the slide mount. Only aqueous-based samples are to be used with these sample wells, No Organic Samples. The sample chambers are single use.
Safety Lids
The safety lids are simply positioned over the four corners of the sample chambers, they are not adhesive. The safety lids stop the expulsion of lysate from the sample chamber which can occur during lysing. Safety lids must always be used during lysing and stop potentially harmful bacterial / viruses from contaminating the slide mount and microwave oven. The safety lids are single use.
A Typical Lysing Procedure
- Pipette the required volume of your sample into the sample chamber.
- Pipette the solution directly between the apex of the triangles, i.e. between the triangles.
-
 Enough aqueous-based sample must be added so that the entire surface within the sample chamber is covered with the fluid to be lysed. Use an appropriate volume sample chamber based on the amount of sample you wish to lyse. 1 ml chambers (0.2 – 1 ml); 3 ml chambers (0.2 – 3 ml) and 5 ml chambers (0.6 – 5 ml).
Enough aqueous-based sample must be added so that the entire surface within the sample chamber is covered with the fluid to be lysed. Use an appropriate volume sample chamber based on the amount of sample you wish to lyse. 1 ml chambers (0.2 – 1 ml); 3 ml chambers (0.2 – 3 ml) and 5 ml chambers (0.6 – 5 ml). - Apply an appropriate safety lid, i.e. 1 & 3 ml or 5 ml lids. Make sure the lid is covering all 4 corners of the sample chamber. Use the “Tag” on the safety lid to position the safety lid. Note: one safety lid is used for both 1 and 3 ml sample chambers.
- Make sure the slide mount is positioned horizontally within the microwave, and then close the microwave oven door.
 On the Microwave control panel, press “Power”, then press “3”, then press “Start”, followed by “3”, “0” “Start”. You will now here the microwave start, and your 30 second lysing procedure has begun. After 30 seconds, the microwave will beep to finish. This setting is 30 % power for 30 seconds.
On the Microwave control panel, press “Power”, then press “3”, then press “Start”, followed by “3”, “0” “Start”. You will now here the microwave start, and your 30 second lysing procedure has begun. After 30 seconds, the microwave will beep to finish. This setting is 30 % power for 30 seconds.- After a few seconds, open the microwave door and remove the Lyse-It slide. Please note, the slide may be very hot, depending on the nature of your sample.
- Remove the safety lid and pipette the Lysate as needed.
- The Lyse-It slide, sample chamber and safety lid should be discarded to your Biological waste.
Cited References
[1] Aslan, K., Previte, M.J.R., Zhang, Y., Gallagher, T., Baillie, L. and Geddes, C.D. (2008). Extraction and Detection of DNA from Bacillus Anthracis Spores and the Vegetative Cells within 1 minute, Analytical Chemistry, 80, 4125-4132.
[2] Zhang, Y., Agreda, P., Kelly, S., Gaydos, C., and Geddes, C. D., (2011). Development of a Microwave-Accelerated Metal-Enhanced Fluorescence 40 seconds, < 100 cfu/ml point of care assay for the detection of Chlamydia Trachomatis. IEEE Transactions on Biomedical Engineering, 58(3), 781-784.
[3] Melendez, J.H., Santaus, T., Brinsley, G., Kiang, D., Mali, B., Hardick, J., Gaydos, C.A., and Geddes, C.D. (2016). Microwave-Accelerated Method for Ultra-Rapid Extraction of Neisseria gonorrhoeae DNA for Downstream Detection. Analytical BioChemistry, 510, 33-40.
[4] Wang, Y., Lee, Y.Y, Santaus, T.M., Newcomb, C.E., Liu, J., Geddes, C.D., Zhang, C., Hu, Q. and Li, Y. (2017). In Situ Enzymatic Conversion of Nannochloropsis oceanica IMET1 Biomass into Fatty Acid Methyl Esters. BioEnergy Research, - In Press. DOI: 10.1007/s12155-016-9807-2.

 Enough aqueous-based sample must be added so that the entire surface within the sample chamber is covered with the fluid to be lysed. Use an appropriate volume sample chamber based on the amount of sample you wish to lyse. 1 ml chambers (0.2 – 1 ml); 3 ml chambers (0.2 – 3 ml) and 5 ml chambers (0.6 – 5 ml).
Enough aqueous-based sample must be added so that the entire surface within the sample chamber is covered with the fluid to be lysed. Use an appropriate volume sample chamber based on the amount of sample you wish to lyse. 1 ml chambers (0.2 – 1 ml); 3 ml chambers (0.2 – 3 ml) and 5 ml chambers (0.6 – 5 ml). On the Microwave control panel, press “Power”, then press “3”, then press “Start”, followed by “3”, “0” “Start”. You will now here the microwave start, and your 30 second lysing procedure has begun. After 30 seconds, the microwave will beep to finish. This setting is 30 % power for 30 seconds.
On the Microwave control panel, press “Power”, then press “3”, then press “Start”, followed by “3”, “0” “Start”. You will now here the microwave start, and your 30 second lysing procedure has begun. After 30 seconds, the microwave will beep to finish. This setting is 30 % power for 30 seconds.